Dr. Reddy P. Kommaddi

Biosketch
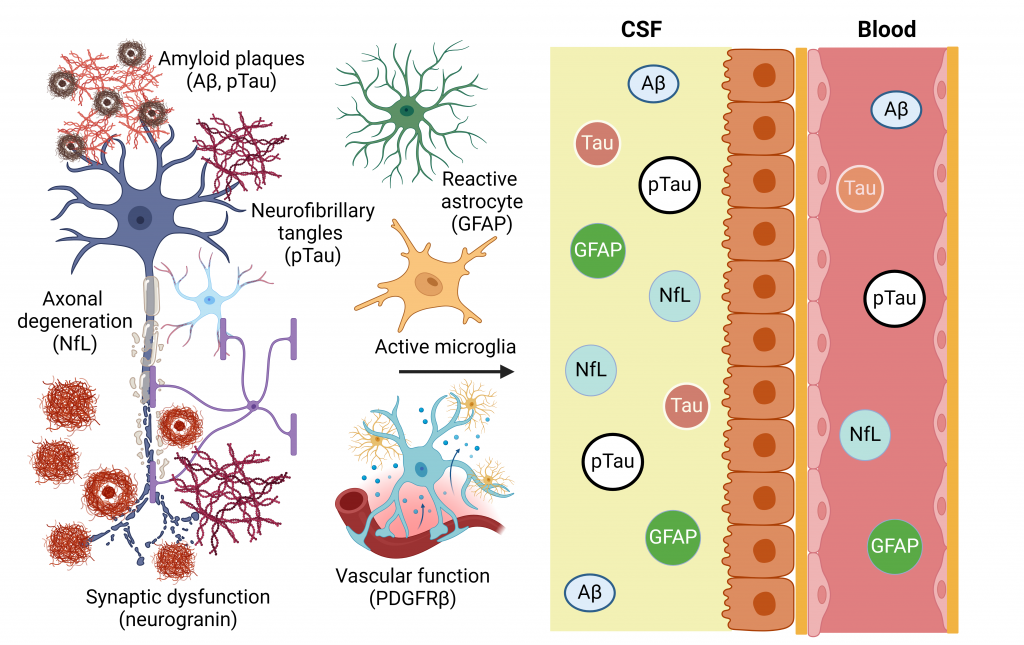
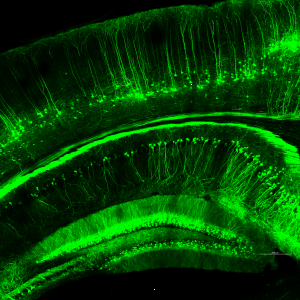
Neuronal projections in the cortex and hippocampus of Thy1-GFP mouse.
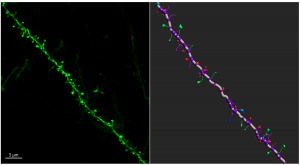
Confocal microscopy image showing the dendrite segment from the cortex. Three-dimensional reconstruction of the dendrite showing the spine morphology.
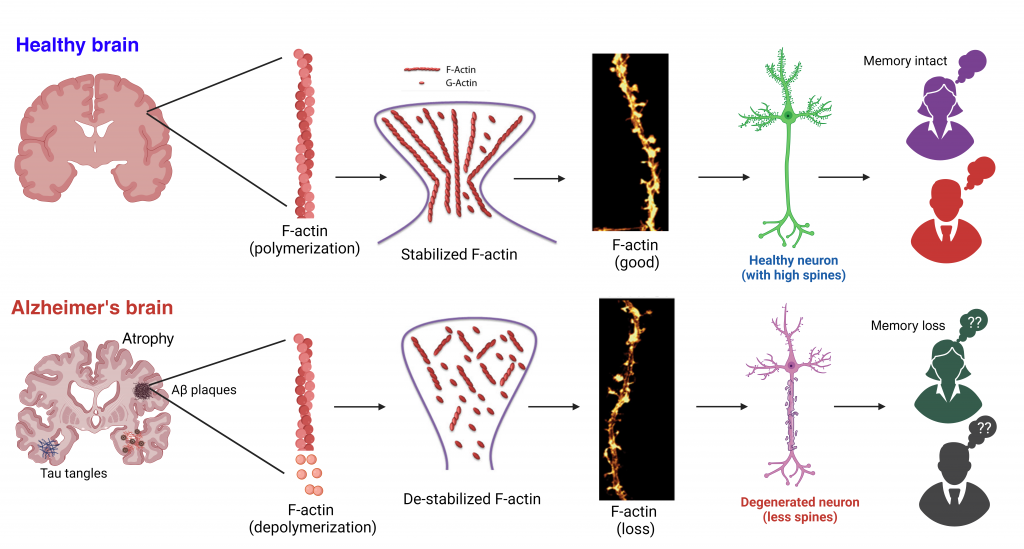
The major risk factors for developing AD are genetics, aging, and female sex. Women account for two-thirds of all people living with AD regardless of age and ethnicity. Sex differences in brain development, adult brain structure, and function have been implicated. Estrogen has long been recognized as a neuroprotective agent. Sex-specific hormones naturally decrease or change with age. Hormone imbalance is associated with synaptic plasticity. We believe that low estrogen levels lead to the exacerbation of AD pathology, deregulation of synaptic function, and perturbation of signaling cascades at the synapse, which ultimately accelerate the AD progression. Therefore, we are primarily focusing on investigating the sex-specific differences through the progression of the disease using AD mouse model. We also want to know how estrogen affects cognitive functions and synaptic functions as well as identify molecular mechanisms and determine potential targets for AD intervention.
Present Members



Alumni

Amrita Mondal
Manasvi
Ramakrishna S, Radhakrishna BK, Kaladiyil AP, Shah NM, Basavaraju N, Freude KK, Kommaddi RP, Muddashetty RS. Distinct calcium sources regulate temporal profiles of NMDAR and mGluR-mediated protein synthesis. Life Sci Alliance. 2024 May 15;7(8):e202402594. PMID: 38749544.
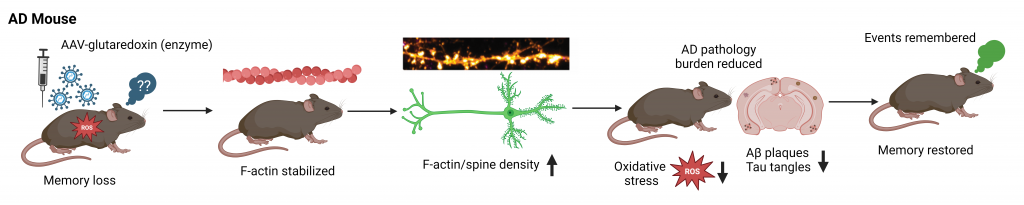
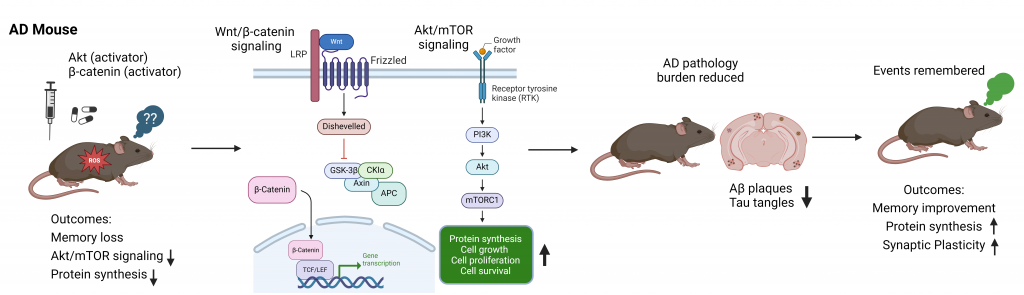
Kommaddi RP, Gowaikar R, Haseena PA, Diwakar L, Singh K, Mondal A. Akt activation ameliorates deficits in hippocampal-dependent memory and activity-dependent synaptic protein synthesis in an Alzheimer’s disease mouse model. J Biol Chem. 2024. 300(2):105619.
Rai P, Sundarakumar JS, Kommaddi RP, Basavaraju N, Issac TG and SANSCOG Study Team. Association between ApoE ?4 genotype and attentional function in non-demented, middle-aged and older adults from rural India. Journal of Neurosciences in Rural Practice. 2023. Doi: 10.25259/JNRP_271_2023.
Kommaddi RP, Verma A, 7. Muniz-Terrera G, Tiwari V, Chithanathan K, Diwakar L, Gowaikar R, Karunakaran S, Malo PK, Graff-Radford NR, Day GS, Laske C, Vöglein J, Nübling G, Ikeuchi T, Kasuga K, the Dominantly Inherited Alzheimer Network (DIAN), Ravindranath V. Sex difference in evolution of cognitive decline: studies on mouse model and the Dominantly Inherited Alzheimer Network cohort. Translational Psychiatry. 2023. 13:123.
Verma A, Kommaddi RP, Gnanabharathi B, Hirsch EC, Ravindranath V. Genes critical for development and differentiation of dopaminergic neurons are downregulated in Parkinson’s disease. J Neural Transm (Vienna). 2023 Feb 23. PMID: 36820885.
Verma A, Ray A, Bapat D, Diwakar L, Kommaddi RP, Schneider BL, Hirsch EC, and Ravindranath V. Glutaredoxin 1 Downregulation in the Substantia Nigra Leads to Dopaminergic Degeneration in Mice. Movement Disorders. 2020. Oct; 35(10):1843-1853.
Kommaddi RP, Deepika ST, Karunakaran S, Bapat D, Nanguneri S, Ray A, Schneider BL, Nair D, Ravindranath V. Glutaredoxin1 diminishes amyloid beta mediated oxidation of F-actin and reverses cognitive deficits in an Alzheimer’s disease mouse model. Antioxid Redox Signal. 2019. Dec 20;31(18):1321-1338.
Ahmad F, Das D, Kommaddi RP, Diwakar L, Gowaikar R, Rupanagudi KV, Bennett DA, Ravindranath V. Isoform-specific hyperactivation of calpain-2 occurs presymptomatically at the synapse in Alzheimer’s disease mice and correlates with memory deficits in human subjects. Sci Rep. 2018 Sep 3;8(1):13119.
Kommaddi RP, Das D, Karunakaran S, Nanguneri S, Bapat D, Ray A, Shaw E, Bennett DA, Nair D, Ravindranath V. Ab mediates F-actin disassembly in dendritic spines leading to cognitive deficits in Alzheimer’s disease. J Neurosci. 2018. Jan 31;38(5):1085-1099.
Jean-Charles PY, Yu S, Abraham D, Kommaddi RP, Mao L, Strachan RT, Zhang ZS, Bowles DE, Brian L, Stiber J, Jones S, Koch WJ, Rockman HA, Shenoy SK. Mdm2 regulates cardiac contractility by inhibiting GRK2-mediated desensitization of b-adrenergic receptor signaling. JCI Insight. 2017; 2(17):e95998.
Ahmad F, Singh K, Das D, Gowaikar R, Shaw E, Ramachandran A, Rupanagudi KV, Kommaddi RP, Bennett DA, Ravindranath V. ROS-mediated loss of synaptic Akt1 signaling leads to deficient activity-dependent protein translation early in Alzheimer’s disease. Antioxid Redox Signal. 2017; 16:1269-1280.
Feger BJ, Thompson JW, Dubois LG, Kommaddi RP, Foster MW, Mishra R, Shenoy SK, Shibata Y, Kidane YH, Moseley MA, Carnell LS, Bowles DE. Microgravity induces proteomics changes involved in endoplasmic reticulum stress and mitochondrial protection. Sci Rep. 2016 Sep 27;6:34091.
Kommaddi RP, Jean-Charles PY, Shenoy SK. Phosphorylation of the deubiquitinase USP20 by protein kinase A regulates post-endocytic trafficking of beta2 adrenergic receptors to autophagosomes during physiological stress. J Biol Chem. 2015 Apr 3;290(14):8888-8903.
Kommaddi RP, Shenoy SK. Arrestins and protein ubiquitination. Prog Mol Biol Transl Sci. 2013;118:175-204. Review.
Han SO, Kommaddi RP, Shenoy SK. Distinct roles for beta-arrestin2 and arrestin-domain-containing proteins in beta2 adrenergic receptor trafficking. EMBO Rep. 2013 Feb;14(2):164-171.
Kommaddi RP, Thomas R, Ceni C, Daigneault K, Barker PA. Trk-dependent ADAM17 activation facilitates neurotrophin survival signaling. FASEB J. 2011 Jun;25(6):2061-70.
Kommaddi RP, Thomas R, Ceni C, Daigneault K, Barker PA. Trk-dependent ADAM17 activation facilitates neurotrophin survival signaling. FASEB J. 2011 Jun;25(6):2061-70.
Kommaddi RP, Dickson KM, Barker PA. Stress-induced expression of the p75 neurotrophin receptor is regulated by O-GlcNAcylation of the Sp1 transcription factor. J Neurochem. 2011 Feb;116(3):396-405.
Ceni C, Kommaddi RP, Thomas R, Vereker E, Liu X, McPherson PS, Ritter B, Barker PA. The p75NTR intracellular domain generated by neurotrophin-induced receptor cleavage potentiates Trk signaling. J Cell Sci. 2010 Jul 1;123(Pt 13):2299-307.
Agarwal V, Kommaddi RP, Valli K, Ryder D, Hyde TM, Kleinman JE, Strobel HW, Ravindranath V. Drug metabolism in human brain: high levels of cytochrome P4503A43 in brain and metabolism of anti-anxiety drug alprazolam to its active metabolite. PLoS One. 2008 Jun 11;3(6):e2337.
Kommaddi RP, Turman CM, Moorthy B, Wang L, Strobel HW, Ravindranath V. An alternatively spliced cytochrome P4501A1 in human brain fails to bioactivate polycyclic aromatic hydrocarbons to DNA-reactive metabolites. J Neurochem. 2007 Aug;102(3):867-877.
Ravindranath V, Kommaddi RP, Pai HV. Unique cytochromes P450 in human brain: implication in disease pathogenesis. Journal of Neural Transmission Suppl. 2006;(70):167-71. Review.
Chinta SJ, Kommaddi RP, Turman CM, Strobel HW, Ravindranath V. Constitutive expression and localization of cytochrome P-450 1A1 in rat and human brain: presence of a splice variant form in human brain. J Neurochem. 2005 May;93(3):724-36.
Pai HV, Kommaddi RP, Chinta SJ, Mori T, Boyd MR, Ravindranath V. A frameshift mutation and alternate splicing in human brain generate a functional form of the pseudogene cytochrome P4502D7 that demethylates codeine to morphine. J Biol Chem. 2004 Jun 25;279(26):27383-9.
-
Centre for Brain Research
Indian Institute of Science Campus
CV Raman Avenue
Bangalore 560012, India. - +91 80 2293 3432
