Dr. Sivaprakasam Ramamoorthy

Education and training:
MSc, Annamalai University, India.
PhD, Medical University of Vienna, Austria.
Postdoc, Duke University Medical Center, North Carolina, USA.
Senior postdoc, Icahn School of Medicine, New York, USA.
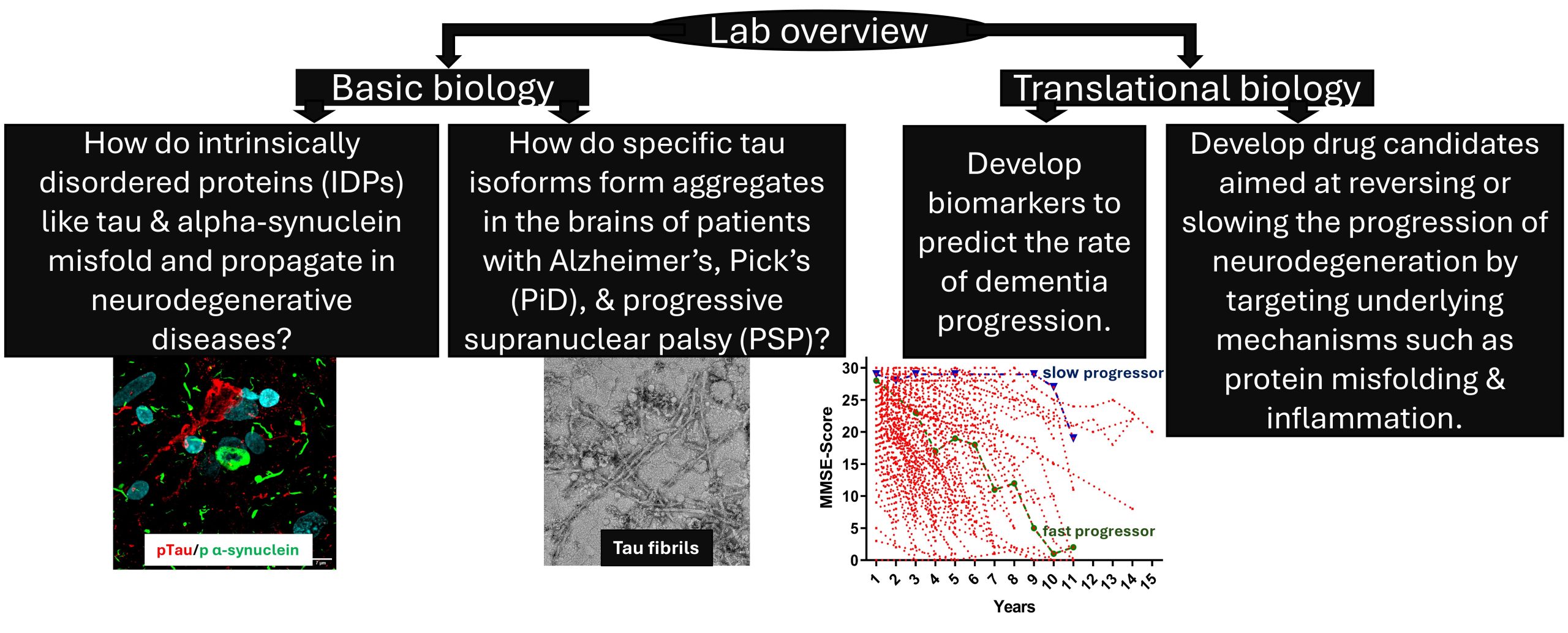
Our laboratory is dedicated to advancing the understanding of neurodegenerative diseases by focusing on the molecular mechanisms underlying protein misfolding and its role in dementia progression. We aim to elucidate the triggers and pathways of tau and alpha-synuclein misfolding, investigate the molecular basis of Alzheimer’s disease progression, and study tau isoform-specific aggregation in disorders like progressive supranuclear palsy (PSP) and Pick’s disease (PiD). Additionally, our research involves the development of drug candidates targeting tauopathies. We are also interested in identifying fluid-based biomarkers to predict and monitor dementia progression. Through these efforts, we aim to contribute to the development of innovative diagnostic and therapeutic strategies for improving patient outcomes in neurodegenerative diseases.
We welcome contributions from potential donors to support our research and industrial partnerships on age-related dementia.
Lab Members

Ms. Preeti Sharma

Mr. Mohammed Waseequr

Mr. Rajath D

Dr. Trisha chattopadhyay

Ms. Aqsa Ellahie

Dr. Ankit Agarwal
Postdoctoral Fellow

Dr. Dev Madhubala
Postdoctoral Fellow
Alumni
Ms. Ayana E K (Project Assistant, 2022-23)
Ms. Nitya Prabhandam (Master’s thesis student, 2023-2024)
Ms. Devika AJ (Intern, 2023-2024)
 Rahman MW, Sharma P, Chattopadhyay T, Saroja SR. Distinct neuronal vulnerability and metabolic dysfunctions are characteristic features of fast-progressing Alzheimer’s patients with Lewy bodies. J Biol Chem. 2025 Mar 10:108396. doi: 10.1016/j.jbc.2025.108396.
Rahman MW, Sharma P, Chattopadhyay T, Saroja SR. Distinct neuronal vulnerability and metabolic dysfunctions are characteristic features of fast-progressing Alzheimer’s patients with Lewy bodies. J Biol Chem. 2025 Mar 10:108396. doi: 10.1016/j.jbc.2025.108396.
 Saroja SR, Gorbachev K, TCW J, Goate AM, Pereira AC. Astrocyte-secreted glypican-4 drives APOE4-depedent tau phosphorylation. Proc Natl Acad Sci U S A. (2022), 119(34):e2108870119.
Saroja SR, Gorbachev K, TCW J, Goate AM, Pereira AC. Astrocyte-secreted glypican-4 drives APOE4-depedent tau phosphorylation. Proc Natl Acad Sci U S A. (2022), 119(34):e2108870119.
 Saroja SR, Sharma A, Hof PR, Pereira AC. Differential expression of tau species and the association with cognitive decline and synaptic loss in Alzheimer’s disease. Alzheimer’s & Dementia (2021), doi:10.1002/alz.12518.
Saroja SR, Sharma A, Hof PR, Pereira AC. Differential expression of tau species and the association with cognitive decline and synaptic loss in Alzheimer’s disease. Alzheimer’s & Dementia (2021), doi:10.1002/alz.12518.
 Kazim SF*, Sharma A*, Saroja SR*, Seo J, Larson CS, Ramakrishnan A, Blitzer RD, Shen L, Peña CJ, Crary JF, Shimoda LA, Nestler EJ, Pereira AC. Chronic intermittent hypoxia enhances pathological tau seeding, propagation, and accumulation, and exacerbates Alzheimer’s-like memory and synaptic plasticity deficits and molecular signatures. Biological Psychiatry (2021), doi:10.1016/j.biopsych. *co-first authors
Kazim SF*, Sharma A*, Saroja SR*, Seo J, Larson CS, Ramakrishnan A, Blitzer RD, Shen L, Peña CJ, Crary JF, Shimoda LA, Nestler EJ, Pereira AC. Chronic intermittent hypoxia enhances pathological tau seeding, propagation, and accumulation, and exacerbates Alzheimer’s-like memory and synaptic plasticity deficits and molecular signatures. Biological Psychiatry (2021), doi:10.1016/j.biopsych. *co-first authors
 Kalaba, P., Aher, N. Y., Ilic, M., Dragacevic, V., … Saroja SR… et al. (2017). Heterocyclic Analogues of Modafinil as Novel, Atypical Dopamine Transporter Inhibitors. Journal of Medicinal Chemistry, 60(22), 9330–9348.
Kalaba, P., Aher, N. Y., Ilic, M., Dragacevic, V., … Saroja SR… et al. (2017). Heterocyclic Analogues of Modafinil as Novel, Atypical Dopamine Transporter Inhibitors. Journal of Medicinal Chemistry, 60(22), 9330–9348.
 Saroja SR, Aher YD, Kalaba P, Aher NY, Zehl M, Korz V, et al (2016). A novel heterocyclic compound targeting the dopamine transporter improves performance in the radial arm maze and modulates dopamine receptors D1-D3. Behav Brain Res 312: 127–137.
Saroja SR, Aher YD, Kalaba P, Aher NY, Zehl M, Korz V, et al (2016). A novel heterocyclic compound targeting the dopamine transporter improves performance in the radial arm maze and modulates dopamine receptors D1-D3. Behav Brain Res 312: 127–137.
 Aher YD, Subramaniyan S, Shanmugasundaram B, Sase A, Saroja SR, Holy M, et al (2016). A Novel Heterocyclic Compound CE-104 Enhances Spatial Working Memory in the Radial Arm Maze in Rats and Modulates the Dopaminergic System. Front Behav Neurosci 10: 20.
Aher YD, Subramaniyan S, Shanmugasundaram B, Sase A, Saroja SR, Holy M, et al (2016). A Novel Heterocyclic Compound CE-104 Enhances Spatial Working Memory in the Radial Arm Maze in Rats and Modulates the Dopaminergic System. Front Behav Neurosci 10: 20.
 Sase A, Aher YD, Saroja SR, Ganesan MK, Sase S, Holy M, et al (2016). A heterocyclic compound CE-103 inhibits dopamine reuptake and modulates dopamine transporter and dopamine D1-D3 containing receptor complexes. Neuropharmacology 102: 186–196.
Sase A, Aher YD, Saroja SR, Ganesan MK, Sase S, Holy M, et al (2016). A heterocyclic compound CE-103 inhibits dopamine reuptake and modulates dopamine transporter and dopamine D1-D3 containing receptor complexes. Neuropharmacology 102: 186–196.
 Karabacak Y, Sase S, Aher YD, Sase A, Saroja SR, Cicvaric A, et al (2015). The effect of modafinil on the rat dopamine transporter and dopamine receptors D1-D3 paralleling cognitive enhancement in the radial arm maze. Front Behav Neurosci 9: 215.
Karabacak Y, Sase S, Aher YD, Sase A, Saroja SR, Cicvaric A, et al (2015). The effect of modafinil on the rat dopamine transporter and dopamine receptors D1-D3 paralleling cognitive enhancement in the radial arm maze. Front Behav Neurosci 9: 215.
 Saroja SR, Kim E-J, Shanmugasundaram B, Höger H, Lubec G (2014). Hippocampal monoamine receptor complex levels linked to spatial memory decline in the aging C57BL/6J. Behav Brain Res 264:1–8.
Saroja SR, Kim E-J, Shanmugasundaram B, Höger H, Lubec G (2014). Hippocampal monoamine receptor complex levels linked to spatial memory decline in the aging C57BL/6J. Behav Brain Res 264:1–8.
 Saroja SR, Sase A, Kircher SG, Wan J, Berger J, Höger H, et al (2014). Hippocampal proteoglycans brevican and versican are linked to spatial memory of Sprague-Dawley rats in the morris water maze. J Neurochem 130: 797–804.
Saroja SR, Sase A, Kircher SG, Wan J, Berger J, Höger H, et al (2014). Hippocampal proteoglycans brevican and versican are linked to spatial memory of Sprague-Dawley rats in the morris water maze. J Neurochem 130: 797–804.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Motivated postdoc candidates with prior experience in molecular biology and protein biochemistry to study protein misfolding mechanisms in neurodegenerative diseases can get in touch with Dr. Sivaprakasam Ramamoorthy- sivar at cbr-iisc.ac.in.
-
Centre for Brain Research
Indian Institute of Science Campus
CV Raman Avenue
Bangalore 560012, India. - +91 80 2293 3748
